If you think that corrosion is just as ugly as the rust of red oxide, it will only cause trouble for steel products and offshore applications in coastal areas, then you are wrong. Corrosion can occur anywhere, even in the driest landlocked countries. Corrosion is also a problem that prevention beforehand is better than solving it afterwards. All we can do is to delay its occurrence and not to eliminate it.

Any metal product, especially those derived from steel, will eventually rust due to contact with oxygen and water and will disintegrate over time.
Two types of reactions occur during the corrosion process: oxidation (metals lose electrons, causing metal corrosion) and reduction reactions (electrons convert water or oxygen into hydroxides). When hydroxide is combined with iron ions, rust is generated. As the metal corrodes, its surface changes, and for ferrous metals, rust can spread to the entire metal surface.
Coastal and offshore applications are more susceptible to rust than other regions because they are exposed to seawater where the pH is neutral or slightly acidic. The products on the shore are in the air with high salt content and are beaten by the tides, leaving residual material on the surface. The closer to the equator, the higher the salt in the air.
However, the occurrence of corrosion is not limited to exposure to seawater. Cleaners, high humidity, and "dirty" environments, such as sewage and mineral extraction, can exacerbate the corrosion process. The chemical production environment contains a lot of carbon dioxide, which also affects metals.
Eva Coronado is the head of the corrosion laboratory at Element Materials Technology in Houston, Texas.
She explained: "Corrosion is a natural phenomenon that occurs in specific humidity, temperature and air conditions; it cannot be avoided and can only be alleviated."
"Corrosion can impair product performance and affect its functionality and integrity. In addition to the increased cost, corrosion can also affect product safety and external aesthetics."
NACE International is the world's largest authority on corrosion research with 30,000 members worldwide. Its former chairman, Kevin Garrity, spent most of his 38-year career in anti-corrosion engineering research. "I was originally an electrical engineer, but I was deeply attracted by corrosion research because it involved many aspects of engineering, such as stress, electrical components, chemical and biological reactions."
He stressed that since humans began to put steel into practical applications, corrosion problems have been with us.
How can we avoid corrosion? The most direct answer is "impossible." The best "protection" approach is to keep the impact of corrosion from the start of the design of the product, taking into account the use of the product and the environment in which it is used, ensuring that materials that are as corrosion-resistant as possible are selected. In addition, the most important thing is that you need to make sure that the metals used do not accelerate the corrosion process because of each other's reactions – this is the well-known Galvanic theory, which is unveiled by the master of electrochemistry, Sir Humphry Davy. When proposed.
According to Galvanic theory, engineers and manufacturers need to align materials and products in a specific way to limit galvanic corrosion. For example, if copper and stainless steel alloys are to be used together, a protective coating is necessary to reduce the occurrence of corrosion. Aluminum alloy and copper should not be used together. If the environment contains salinity and the pH is high, it should be especially avoided. In addition, it should always be borne in mind that galvanic differences between materials can be affected by the environment in which the application or product is located.
Lack of awareness of the galvanic reaction can have devastating effects on the economy and safety and damage the company's image. A major accident at a refinery in the United States was caused by corrosive cracks caused by corrosion, and its economic loss was about $500 million.
Garrity saw a lot of dangerous galvanic reactions during his NACE career, and he even remembered the accident at a nuclear power plant in the United States. "The factory's copper grounding system was originally designed to ensure the safety of personnel and equipment in the event of an electrical fault, but the system is connected to the hydrophobic piping system to form a battery-like reaction. Due to the reaction between copper and bismuth, The pipe will eventually corrode copper, causing leakage, which will result in a small amount of radioactive material leaking."
There are some "standards" that can delay the corrosion process of a product or application: selecting a material with similar latitude potential; using a special paint or coating to create a protective barrier; using a sacrificial anode to protect the core product; or introducing a current to counteract any galvanic reaction.
The type of corrosion protection used depends on the type of metal, the actual application, the application environment, and the capital budget that the company plans to invest.
Protective coatings are the standard and lowest cost corrosion protection method, but not foolproof. For example, some protective coatings can enhance the corrosion resistance of articles in seawater environments, but they cannot withstand the corrosion caused by degreasing solutions.
Environmentally friendly anti-corrosion coatings are also widely controversial, especially in the automotive industry. Some believe that it is best to use hard anti-corrosion coatings in a sealed environment, because products that use this anti-corrosion coating last three times longer than products that use environmentally friendly coatings, while parts that use environmentally friendly coatings need to be replaced over their useful life. three times.
Another method of resisting galvanic reactions is to introduce a sacrificial anode metal such as a block, rod, disk or ribbon of magnesium, aluminum or zinc to protect the metal structure or application product. The metal acts as a cathodic protection device to prevent corrosion of the major part of the structure by absorbing the oxidation reaction. This method requires an electron path between the anode and the metal (such as connecting or bringing the wires together), and between the oxidant (such as water or wet soil) and the anode, and between the oxidant and the metal to be protected. There must be an ion channel to form a closed loop.
Magnesium, aluminum and zinc are the most common sacrificial anode materials. Lighter weight aluminum is commonly used in offshore and offshore applications (such as hulls, offshore pipelines, and storage tanks), but it can not be used in flammable or explosive environments because it can spark when in contact with rusted surfaces. As the anode metal with the highest negative potential, magnesium is often used in underground and land-related applications.
Many corrosion conditions can be avoided if adequate measures are taken during the product design and development phase. Kevin Garrity pointed out: "At NACE, we train about 12,000 engineers every year on corrosion prevention. However, there are more than 3.5 million engineers worldwide, so there are still many people who need training."
Garrity said that many companies and industry organizations are beginning to realize that while preventing corrosion will bring more cost in the early stages, it will save money in the long run.
He suggested calculating the return on the investment by establishing a risk model and “sorting the threats to potential corrosion risks based on the importance of the structure or facility, and then eliminating the risk factors in sequence.â€
Bolts are only a small part of the entire construction process, but they still need to be carefully designed. If the bolt is corroded, regardless of the size of the building or product, there is a risk of collapse.
Coronado said: "Fasteners are an indispensable tool in modern life and must be stable and reliable. Corrosion of fasteners not only causes metal loss, but also can cause fastener failure and even cause high-strength fastener cracking and sudden failure. Since corrosion-resistant fasteners are not suitable for all situations, other methods of mitigating corrosion, such as anti-corrosion coatings, are also used to protect the fasteners."
Zinc coatings, such as Delta Protekt® or Delta-Tone®, are the most commonly used coatings for protecting steel bolts and gaskets. This kind of paint is used like paint and forms a protective film after baking. If it is applied in multiple layers, it can also prevent friction. Other options include Teflon coatings and hot dip galvanizing.
Franz Raymann, head of technical services at Nord-Lock, believes that choosing the right material for the bolts and using proper corrosion protection is critical. He said: "Customers often don't understand why we ask so many questions, why we need to understand the materials they use and the application environment. But we can only provide the right bolts if we have the details."
For example, he said that an offshore platform weighing 4,000 tons stood on the surface with four pillars and was fixed by 16 giant bolts. "If these bolts are corroded, all the workers on the platform will be buried in the North Sea."
"The general strategy is to periodically check the corrosion of the bolts. If corrosion is found during the inspection, remove and clean it according to the severity, check for cracks and reapply the anti-corrosion coating, or replace the entire bolt directly."
How to resist corrosion by design
Analyze the environment and conditions required for corrosion to occur.
Choose materials that have sufficient corrosion resistance (and similar galvanic electrodes).
Avoid the use of geometries that tend to accumulate moisture and dirt, cause stress concentration, and cause erosion.
Choose the appropriate corrosion protection method (surface coating, sacrificial anode, direct current, etc.).
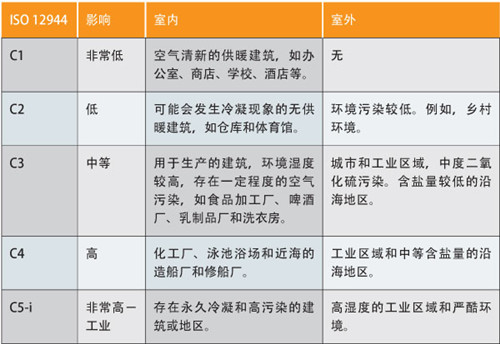
Clear requirements: for example, ISO 9227 salt spray corrosion test, ASTM G48 electrochemical corrosion test for stainless steel materials, ISO 12944 environmental corrosion category.
Classification of corrosive environments
We have been in the research, development, sales of different pharmaceutica Intermediate and organic intermediate chemicals since our foundation, severing customs in the US, Japan, South East Asia, South America, Middle East etc.
For example, sodium bromide is used for making sensitive emulsion of photographic film and for making medicinal intermediates in medico-industries, e.g. diuretic , sedative etc. It is also used as bromide making complex material and complex dyes.Characters: Colorless cubic crystal or white granular powder. Odourless, taste salty and bitter, soluble in water, slightly soluble in alcohol.Packed in 25kg bag.
Intermediate
Diphenylacetonitrile Pharm Intermediate,Betaine Citrate Pharmaceutical Intermediate,Pesticide Intermediates,Medicine Intermediate
Gemhold (SJZ) Trading Co., Ltd. , https://www.gemhold.cn